-
Vibrio parahaemolyticus(V. parahaemolyticus) is a Gram-negative, halophilic bacterium found globally in environments such as oceans, rivers, and seabed sediments (1). Infections withV. parahaemolyticusare caused by diverse serotypes, with O3:K6 reported as the most dominant (2–5). In China,V. parahaemolyticusis the most common foodborne pathogen causing sporadic foodborne disease in coastal areas and the leading etiologic agent of foodborne disease outbreaks (6). However, most epidemiological studies onV. parahaemolyticushave focused on foodborne disease outbreaks (7–9), with few studies based on long-term, continuous, and systematic sentinel active surveillance.
-
This study was conducted from 2013 to 2022 under the framework of the national foodborne disease active surveillance program in 31 provincial-level administrative divisions (PLADs) in China. The China National Center for Food Safety Risk Assessment (CFSA) maintains and manages all national foodborne disease surveillance data and systems. According to the national foodborne disease surveillance plan, sentinel hospitals are responsible for collecting epidemiological information, clinical signs and symptoms, suspected food exposure information, and stool specimens from patients with diarrhea to test forSalmonella,Shigella,V. parahaemolyticus, diarrheagenicEscherichia coli, and norovirus, and to undertake identification and serotyping of positive strains. Cases positive forV. parahaemolyticuswere selected for further analysis in this study. Data cleaning and database creation were performed using Microsoft Office (version 2010, Microsoft, Washington, USA), and SPSS (version 22.0, SPSS Inc, Chicago, USA) was used for statistical analysis. All variables were presented as counts or percentages. All procedures involving human participants followed the ethical standards of the ethics committee of Ningbo Municipal Center for Disease Control and Prevention and were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards (Approval No.: 202204).
-
From 2013 to 2022, the National Foodborne Disease Case Surveillance System documented 23,818 cases ofV. parahaemolyticusdiarrhea. Patient ages ranged from 2 months to 100 years (mean: 38.41 years), with 12,126 cases (50.91%) occurring in individuals aged 24–44 years. The most common symptoms were watery diarrhea (22,642 cases, 95.06%), abdominal pain (16,381 cases, 68.78%), nausea (11,158 cases, 46.85%), vomiting (9,593 cases, 40.28%), and fever (3,281 cases, 13.78%).
Analysis of the overallV. parahaemolyticusdetection rate in sporadic diarrhea cases in China revealed a rate of 1.83% (23,818/1,298,516). Among the top 5 PLADs with the highest detection rates, Shanghai had the highest rate at 5.50% (2,352/42,759), followed by Zhejiang (3.45%, 11,751/340,354), Liaoning (3.43%, 1,054/30,711), Beijing (3.26%, 1,693/51,900), and Hainan (3.14%, 503/16,030). The 5 PLADs with the highest number of cases were Zhejiang (11,751 cases), Jiangsu (2,471 cases), Shanghai (2,352 cases), Beijing (1,693 cases), and Liaoning (1,054 cases), respectively. These PLADs accounted for 49.34% (11,751/23,818), 10.37% (2,471/23,818), 9.87% (2,352/23,818), 7.11% (1,693/23,818), and 4.43% (1,054/23,818) of the total cases. The distribution of detection rates and case numbers across these regions is illustrated inFigure 1.
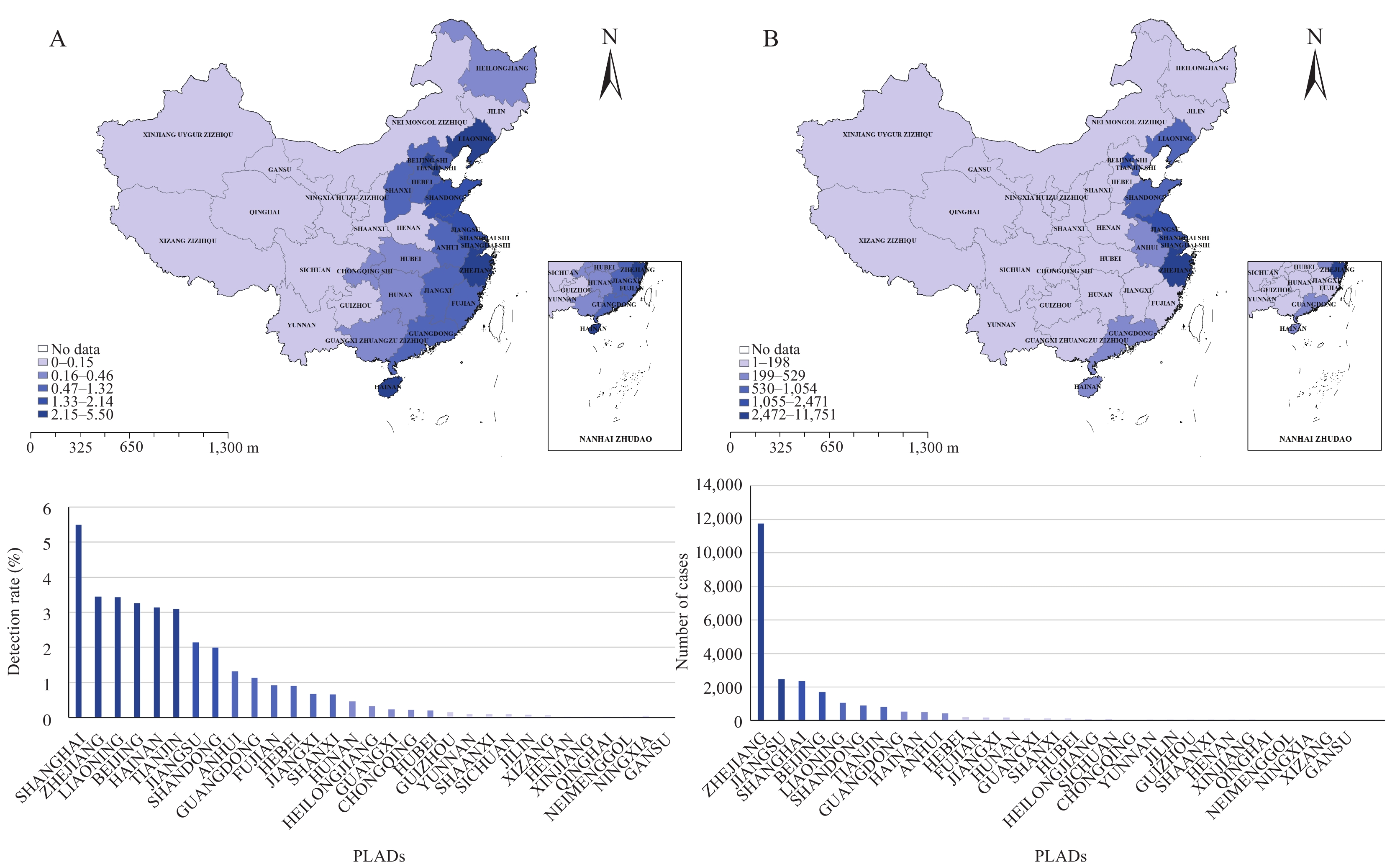 Figure 1.
Figure 1.Detection rate and number of cases of foodborne disease caused byV. parahaemolyticusin China from 2013 to 2022. (A) Detection rate; (B) Case number.
Note: Map approval number: GS 京(2024)1947号.Of the 23,818 foodborne disease cases caused byV. parahaemolyticus, 78.20% occurred between July and September. Using the circular distribution test (10), the peak occurrence ofV. parahaemolyticus-induced foodborne disease in China from 2013 to 2022 was determined to be August 12, with a peak period from July 3 to September 21. Analysis of the peak disease periods in 12 coastal PLADs with high disease incidence revealed a distinct pattern. Regions with higher latitudes and lower temperatures tended to have peak periods that started later, ended earlier, and were shorter in duration. Conversely, regions with lower latitudes and higher temperatures exhibited peak periods that started earlier, ended later, and lasted longer. Among the 12 coastal PLADs, Liaoning Province experienced the shortest peak period forV. parahaemolyticus-related diseases, from July 13 to August 24. In contrast, Hainan Province recorded the longest peak period, extending from June 14 to October 20. The time distribution and peak periods of cases from 2013 to 2022 are shown inFigure 2.
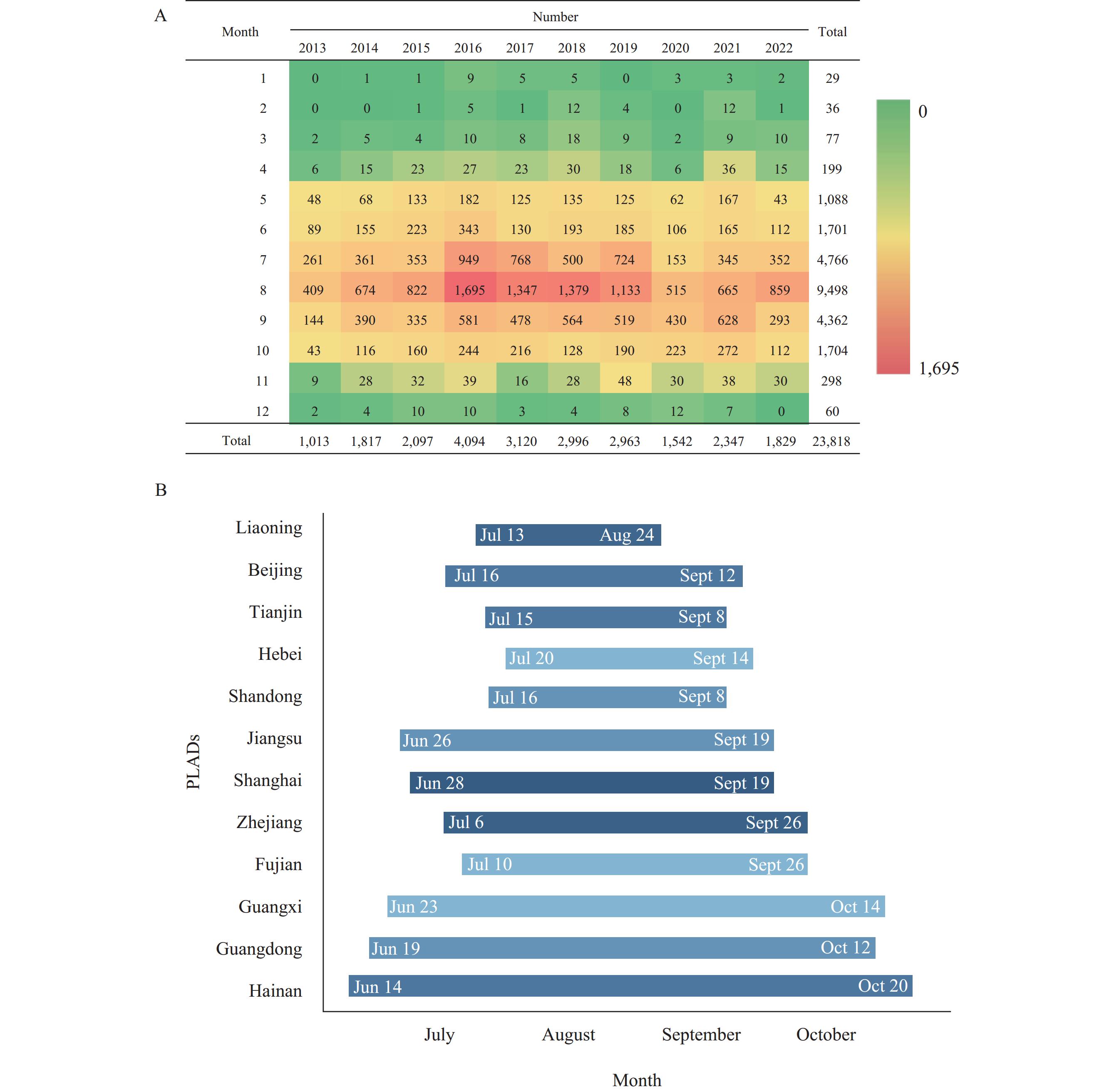 Figure 2.
Figure 2.Time distribution and peak periods of foodborne disease caused byV. parahaemolyticusin China from 2013 to 2022. (A) Time distribution; (B) Peak periods.
A total of 81.88% of patients reported consuming suspicious foods, primarily aquatic animals and their derivatives (34.37%, 6,704/19,503). Meat and meat products were the second most common category (17.99%, 3,509/19,503), followed by various other foods (9.24%, 1,802/19,503).
Among the 23,818 isolates from 2013 to 2022, 11,987 strains ofV. parahaemolyticuswere serotyped for somatic (O) antigen, achieving a serotyping rate of 50.33%. The major serotypes identified were O3 and O4, comprising 49.59% and 23.25%, respectively.Table 1revealed that 7,342 strains were further serotyped for both somatic (O) and capsular (K) antigens, accounting for a serotyping rate of 30.83%. Among these, the most prevalent serotypes were O3:K6 and O10:K4, representing 55.03% and 23.86%, respectively. Serotype O10:K4 emerged only in 2020 and became dominant in China in 2021–2022.
Serotype Number (%) 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 Total O3:K6 104
(69.33)410
(79.77)425
(83.66)925
(74.96)225
(74.26)150
(79.37)957
(79.95)401
(58.37)209
(13.91)234
(22.12)4040
(55.03)O10:K4 0
(0)0
(0)0
(0)0
(0)0
(0)0
(0)0
(0)139
(20.23)1039
(69.17)574
(54.25)1752
(23.86)O4:K8 37
(24.67)56
(10.89)61
(12.01)164
(13.29)45
(14.85)18
(9.52)117
(9.77)44
(6.40)31
(2.06)20
(1.89)593
(8.08)O4:K4 8
(5.33)1
(0.19)0
(0)0
(0)7
(2.31)7
(3.70)1
(0.08)8
(1.16)64
(4.26)68
(6.43)164
(2.23)O3:K4 0
(0)0
(0)0
(0)0
(0)0
(0)3
(1.59)1
(0.08)24
(3.49)43
(2.86)21
(1.98)92
(1.25)O1:K1 0
(0)1
(0.19)1
(0.20)14
(1.13)0
(0)1
(0.53)29
(2.42)6
(0.87)11
(0.73)18
(1.70)81
(1.10)O4:K6 0
(0)1
(0.19)8
(1.57)46
(3.73)4
(1.32)0
(0)1
(0.08)4
(0.58)0
(0)2
(0.19)66
(0.90)O1:K4 0
(0)0
(0)0
(0)0
(0)2
(0.66)0
(0)0
(0)0
(0)7
(0.47)23
(2.17)32
(0.44)O10:K60 0
(0)17
(3.31)2
(0.39)6
(0.49)0
(0)1
(0.53)0
(0)0
(0)4
(0.27)1
(0.09)31
(0.42)O10:K24 0
(0)0
(0)0
(0)1
(0.08)0
(0)0
(0)0
(0)9
(1.31)15
(1.00)1
(0.09)26
(0.35)O1:K56 0
(0)2
(0.39)1
(0.20)4
(0.32)1
(0.33)0
(0)4
(0.33)2
(0.29)6
(0.40)5
(0.47)25
(0.34)O2:K5 0
(0)0
(0)0
(0)0
(0)0
(0)0
(0)15
(1.25)9
(1.31)1
(0.07)0
(0)25
(0.34)O1:K3 1
(0.67)0
(0)2
(0.39)1
(0.08)4
(1.32)0
(0)6
(0.50)1
(0.15)2
(0.13)3
(0.28)20
(0.27)O1:K6 0
(0)1
(0.19)0
(0)5
(0.41)1
(0.33)0
(0)4
(0.33)4
(0.58)0
(0)5
(0.47)20
(0.27)O1:K36 0
(0)7
(1.36)0
(0)5
(0.41)0
(0)0
(0)3
(0.25)2
(0.29)1
(0.07)1
(0.09)19
(0.26)O1:K25 0
(0)0
(0)1
(0.20)0
(0)0
(0)0
(0)13
(1.09)2
(0.29)1
(0.07)0
(0)17
(0.23)O4:K68 0
(0)0
(0)1
(0.20)1
(0.08)0
(0)0
(0)7
(0.58)0
(0)8
(0.53)0
(0)17
(0.23)O2:K3 0
(0)0
(0)0
(0)5
(0.41)0
(0)3
(1.59)6
(0.50)0
(0)0
(0)2
(0.19)16
(0.22)O1:K5 0
(0)1
(0.19)1
(0.20)1
(0.08)2
(0.66)0
(0)0
(0)2
(0.29)3
(0.20)5
(0.47)15
(0.20)O4:K9 0
(0)0
(0)1
(0.20)0
(0)0
(0)2
(1.06)2
(0.17)2
(0.29)3
(0.20)1
(0.09)11
(0.15)O3:K8 0
(0)1
(0.19)1
(0.20)4
(0.32)3
(0.99)0
(0)1
(0.08)0
(0)0
(0)0
(0)10
(0.14)O4:K55 0
(0)2
(0.39)0
(0)2
(0.16)0
(0)0
(0)0
(0)0
(0)4
(0.27)2
(0.19)10
(0.14)O8:K4 0
(0)0
(0)0
(0)0
(0)0
(0)0
(0)0
(0)0
(0)1
(0.07)9
(0.85)10
(0.14)Others 0
(0)14
(2.72)3
(0.59)50
(4.05)9
(2.97)4
(2.12)30
(2.51)28
(4.08)49
(3.26)63
(5.95)250
(3.41)Total 150
(100.00)514
(100.00)508
(100.00)1,234
(100.00)303
(100.00)189
(100.00)1,197
(100.00)687
(100.00)1,502
(100.00)1,058
(100)7,342
(100)Table 1.Distribution of main serotypes ofV. parahaemolyticusfrom 2013 to 2022.
-
This study examined the epidemiological characteristics of foodborne diseases caused byV. parahaemolyticusin China using active surveillance data from 2013 to 2022. A total of 23,818 cases ofV. parahaemolyticusfoodborne illness were identified and collated through the national foodborne disease surveillance system. Distinct monthly peaks in cases were observed during warmer months, particularly from July to September. Coastal regions accounted for most cases, with a significantly higher detection rate ofV. parahaemolyticusthan inland areas. Analysis of the 23 serotypes detected revealed the diversity ofV. parahaemolyticus, with serotypes O3:K6 and O10:K4 being the most prevalent. Notably, serotype O10:K4 has become increasingly prominent, surpassing O3:K6 as the dominant serotype in China since 2021.
This seasonality and coastal distribution ofV. parahaemolyticusinfection have also been observed in other countries. This phenomenon is thought to be associated with the thermophilic nature ofV. parahaemolyticus, as coastal residents have a higher risk of consuming raw, undercooked, or mishandled seafood (11). The duration of the disease peak varies by region, mainly related to latitude and local temperature conditions, which may affect the growth and reproduction ofV. parahaemolyticus. Recent studies have also shown a link between the appearance ofVibrioepidemic outbreaks and environmental factors, such as the oceanic transport of warm waters, providing a possible mechanism for the global dispersion ofVibriodiseases. In general, younger patients, particularly those under 5 years of age, tend to experience a higher frequency and variety of viral infections, while adults aged 18 to 45 are more susceptible to bacterial pathogens (12). This study agreed with this trend, as more than half of theV. parahaemolyticuscases occurred in the 24-44-year-old age group. This age distribution pattern could indicate a natural evolution in host immunity and/or dietary preferences that correlate with age. This study also revealed that the incidence of foodborne illnesses declined significantly during the COVID-19 pandemic. This decline may be attributed to policies, restrictions, and environmental and behavioral changes.
Frequent recombination events around the O- and K-antigen coding gene cluster inV. parahaemolyticuscan lead to serotype transformation (13–15). In recent years, the serotype O10:K4 has become increasingly prevalent, surpassing O3:K6 as the dominant serotype in China. This suggests that newV. parahaemolyticusantigens may emerge and spread in the future.
This study was subject to some limitations. First, due to differing detection capabilities in sentinel hospitals, this study may not fully reflect the real-world epidemiological characteristics of foodborne disease caused byV. parahaemolyticus. Second, the actual number of foodborne disease cases may be larger than the number of monitored cases. This discrepancy may arise because foodborne disease often self-resolves, receives limited patient attention, and is prone to underreporting in the monitoring network. Third, the serotyping rate for both O and K antigens is relatively low and may not represent the true prevalence ofV. parahaemolyticusserotypes. Fourth, this study did not investigate the virulence factors ofV. parahaemolyticus.
Based on the study results, food regulatory authorities should intensify routine surveillance and inspections targeting high-risk periods and locations. Oversight of production, marketing enterprises, and catering services should be strengthened, with a focus on crucial steps such as raw seafood processing and storage. Additionally, relevant departments should leverage social media to promote public awareness and education on preventing and controllingV. parahaemolyticus-related foodborne illnesses. This will improve public knowledge and self-protection regarding these illnesses.
-
All the sentinel hospitals and Centers for Disease Control and Prevention in 31 PLADs for their enthusiastic participation in the foodborne disease active surveillance.
HTML
| Citation: |




 Download:
Download:





